- 导读
近年来,锂硫电池中涉及多电子反应的复杂硫转化过程所引发的穿梭效应和动力学迟滞,已成为严重阻碍其活性物质利用率与循环寿命提升、制约商业化进程的核心科学问题。针对此瓶颈,本研究创新性地将肖特基型氧空位(S-Ovs)工程应用于p区氧化铋(Bi₂O₃)催化剂。通过湿法还原技术在负载于木质素碳纳米纤维的Bi₂O₃上精准构建肖特ky型氧空位,有效降低Bi的p带中心,增强反键轨道电子占据,从而实现对LiPSs的适中化学吸附,显著降低了反应能垒并加速了转化动力学。
二、成果掠影
近日,莆田学院易明杰教授,清华大学杨洋博士,大连工业大学胡顺友和孙润仓教授联合地提出了一种肖特基型氧空位(S-Ovs)介导的轨道工程策略,用以精准调控p区氧化铋(Bi₂O₃)的电子结构,从而大幅提升锂硫电池的催化转化动力学。理论计算与原位表征共同揭示,该策略能有效降低Bi的p带中心(-3.7 eV),优化其对多硫化物的吸附强度,并显著降低液-固转化步骤的反应能垒。基于此材料构建的功能性隔膜,使得锂硫电池表现出卓越的综合性能:在1 C倍率下循环1000次,容量衰减率仅为0.032%/圈;即使在高硫负载(7.0 mg cm⁻²)和贫电解液条件下,仍能实现4.36 mAh cm⁻²的高面容量。该工作为通过缺陷工程调控p区金属轨道以设计高效催化剂提供了新范式。
该成果以莆田学院为第一单位,大连工业大学第二单位,清华大学第三单位以题目“Schottky-Type Oxygen Defect and Orbital Engineering of Bismuth Oxide for Boosting Sulfur Redox Kinetics”发表在SCI/中科院一区top期刊Energy Storage Materials上,if为20.2。
三、核心创新点
本工作的核心创新在于首次将肖特基型氧空位作为一种高效的“电子编辑器”,用于精准调控p区铋基催化剂的电子结构。通过简单的湿法还原策略,不仅成功构筑了富含肖特基缺陷的结构,更关键的是实现了对铋原子6p轨道电子占据数(Bi 6s²6pθ, 0<θ<3)和p带中心的连续、精准调控。这一独特的轨道工程使其对多硫化物的吸附能遵循“萨巴蒂尔原理”,达到最佳平衡点,从而显著加速了硫还原反应动力学,特别是液-固转化这一决速步骤。这为超越传统d区金属、设计新一代p区高效催化剂提供了全新范式。
四、数据概览
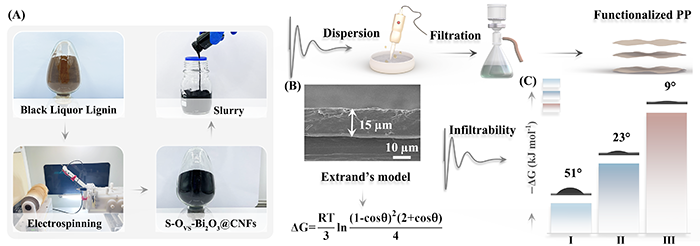
Fig. 1. (A) Digital images of the functional separator preparation process. (B) SEM image of the S-OVS-Bi2O3@CNFs/PP separator. (C) The contact angle and wetting Gibbs energy of separators (Ⅰ: PP, Ⅱ: Bi2O3@CNFs, Ⅲ: S-OVS-Bi2O3@CNFs).
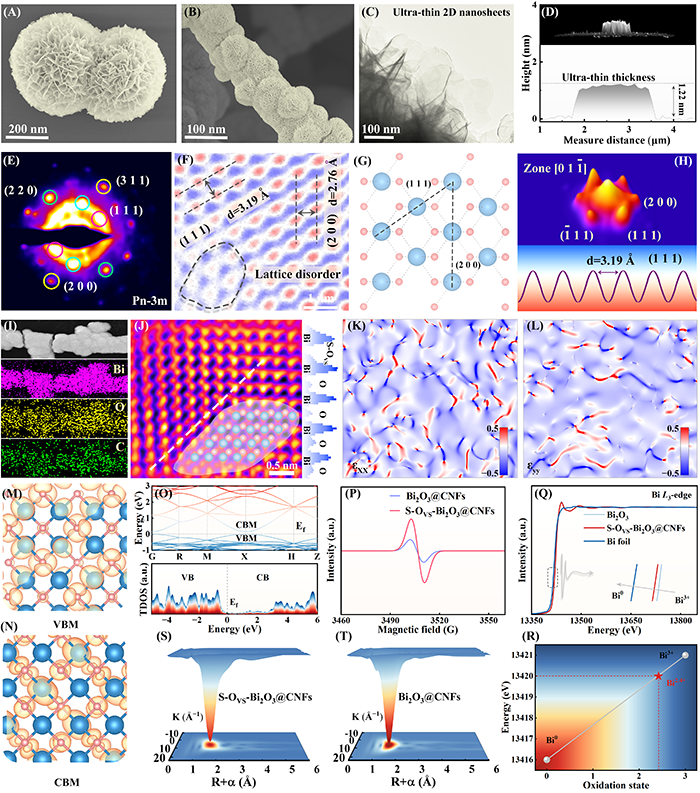
Fig. 2. SEM images of (A) Bi2O3 and (B) S-OVS-Bi2O3@CNFs. (C) TEM and (D) AFM images of S-OVS-Bi2O3@CNFs. (E) Selected-area electron diffraction pattern. (F, J) Spherical aberration-corrected HAADF-STEM images of S-OVS-Bi2O3@CNFs and corresponding (G) crystal structure and (H) the FFT pattern. (I) Elemental mapping images of S-OVS-Bi2O3@CNFs. (K, L) Geometric phase analysis of S-OVS-Bi2O3@CNFs. Spatial charge distribution of the (M) VBM and (N) CBM of S-OVS-Bi2O3. (O) Energy band diagram and DOS of S-OVS-Bi2O3. (P) EPR results of Bi2O3 and S-OVS-Bi2O3@CNFs. (Q) Bi L3-edge XANES spectra. (R) Relationship between L3-edge absorption energy and oxidation state. Wavelet transform contour plots of (S) S-OVS-Bi2O3@CNFs and (T) Bi2O3@CNFs.
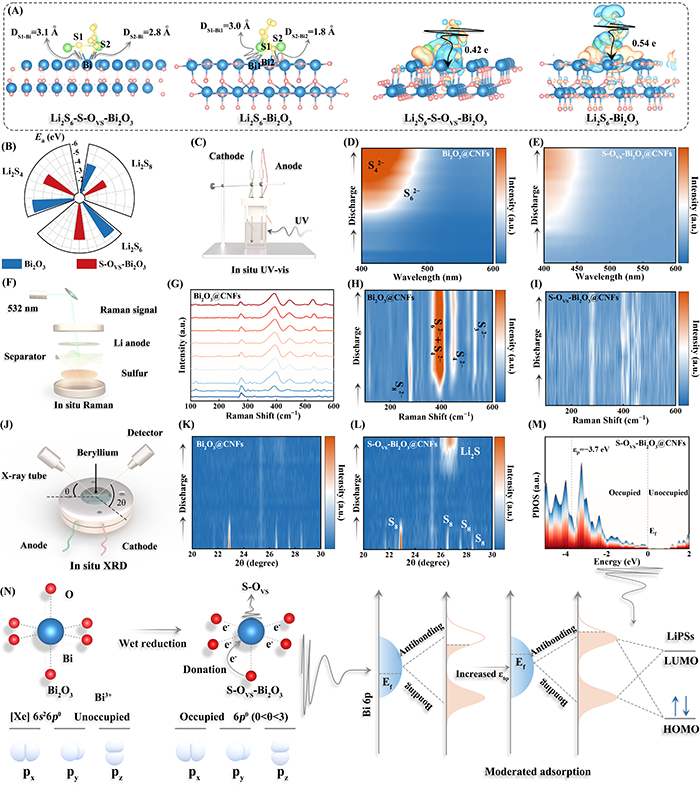
Fig. 3. (A) Optimized structures of Li2S6 adsorbed on S-OVS-Bi2O3 and Bi2O3, along with the corresponding charge density differences. (B) The adsorption energies between LiPSs and catalysts. (C) Schematic illustration of in situ UV-vis and corresponding (D, E) spectra. (F–I) Schematic illustration of in situ Raman and corresponding spectra. (J) Schematic illustration of in situ XRD and corresponding (K, L) spectra. (M) p-band center of S-OVS-Bi2O3. (N) Schematic diagram of the relationship between the 6p orbital electron configuration and adsorption energy.
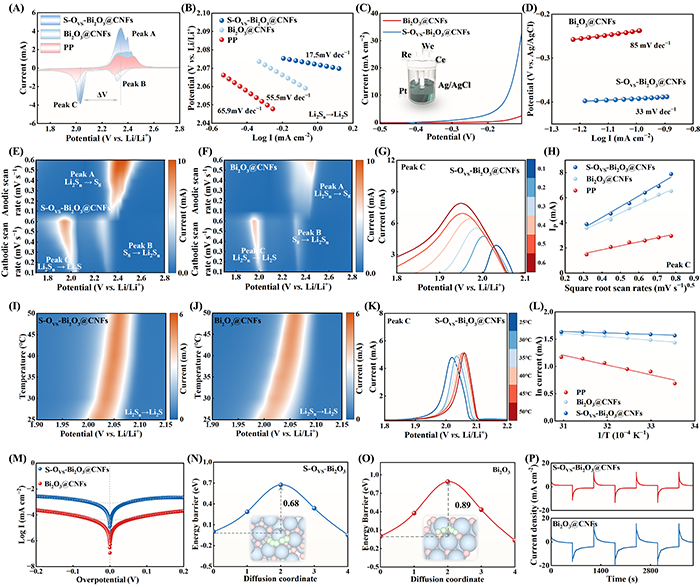
Fig. 4. (A) CV profiles and corresponding (B) Tafel plots (LiPSs→Li2S). (C) LSV curves and corresponding (D) Tafel plots. Contour plots of CV patterns of (E) S-OVS-Bi2O3@CNFs and (F) Bi2O3@CNFs. (G) Enlarged peak C in the CV curves of S-OVS-Bi2O3@CNFs. (H) Fitting line between IP and v0.5. Contour plots of the CV patterns of (I) S-OVS-Bi2O3@CNFs and (J) Bi2O3@CNFs at different temperatures. (K) Enlarged peak C in the CV curves. (L) Fitting line between lnI and 1/T. (M) Tafel plots of Li2S6 symmetric cells. Energy barrier for Li+ migration within (N) S-OVS-Bi2O3 and (O) Bi2O3. (P) Chronoamperometric curves of the Li2S6 symmetric cells.
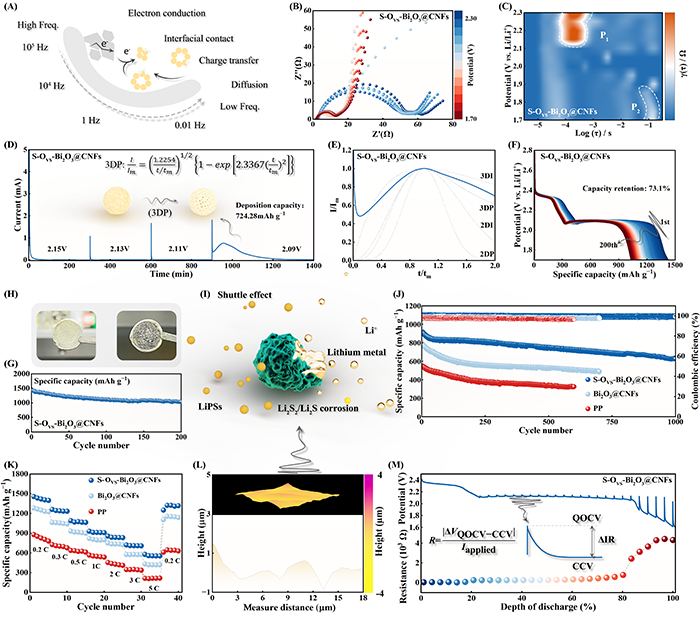
Fig. 5. (A) Schematic depiction of the electrochemical behavior of Li||S batteries across various frequency ranges. (B) In situ EIS analysis and corresponding (C) DRT plot during the discharge process. (D) Kinetic evaluation of Li2S nucleation via PITT measurement. (E) Dimensionless current-time transient during Li2S nucleation. (F) Galvanostatic discharge profiles of the S-OVS-Bi2O3@CNFs-based cell and the corresponding (G) cycling performance. (H) Photographs of the Li anodes after cycling (left: S-OVS-Bi2O3@CNFs; right: CNFs). (I) Schematic representation of Li anode corrosion caused by the shuttle effect. (J) Cycling performance of the batteries at a rate of 1.0 C. (K) Rate capability of the Li||S batteries. (L) AFM images of the Li anode in the S-OVS-Bi2O3@CNFs-based cell. (M) GITT curves and corresponding resistance of the S-OVS-Bi2O3@CNFs-based cell.
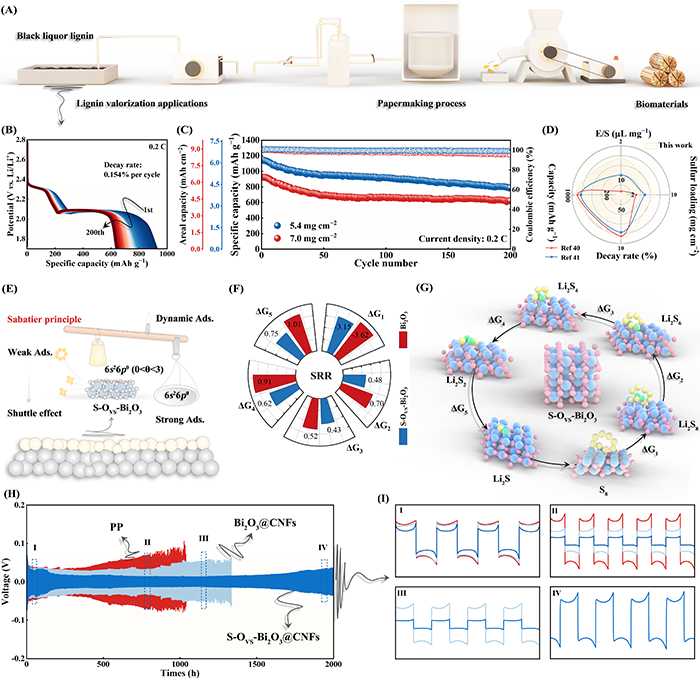
Fig. 6. (A) Schematic illustration of the high-value utilization of black liquor lignin. (B) Discharge curves and corresponding (C) cycling performance of S-OVS-Bi2O3@CNFs-based cells with high sulfur loadings. (D) Comparison of Li||S batteries with high sulfur loadings in previous studies. (E) Schematic illustration of S-OVS-Bi2O3@CNFs enhancing Li||S batteries. (F, G) Gibbs free energy for SRR. (H, I) Cycling performance of Li||Li symmetric cells.
五、成果启示
通过将缺陷工程的应用从传统的d区过渡金属拓展至p区主族金属,揭示了通过构筑肖特基型氧空位来精准调控金属p轨道电子结构,从而实现催化性能优化的普适性新路径。这一“轨道工程”策略为解决p区金属催化剂普遍存在的活性调控难题提供了关键理论依据和实践范例。更重要的是,该工作将生物质衍生碳(木质素)与精细的电子结构调控相结合,展示了将低成本、可持续的原材料转化为高性能储能器件的巨大潜力,为面向实际应用的高性能、低成本锂硫电池的设计开发提供了从基础理论到材料创新的全链条解决方案。